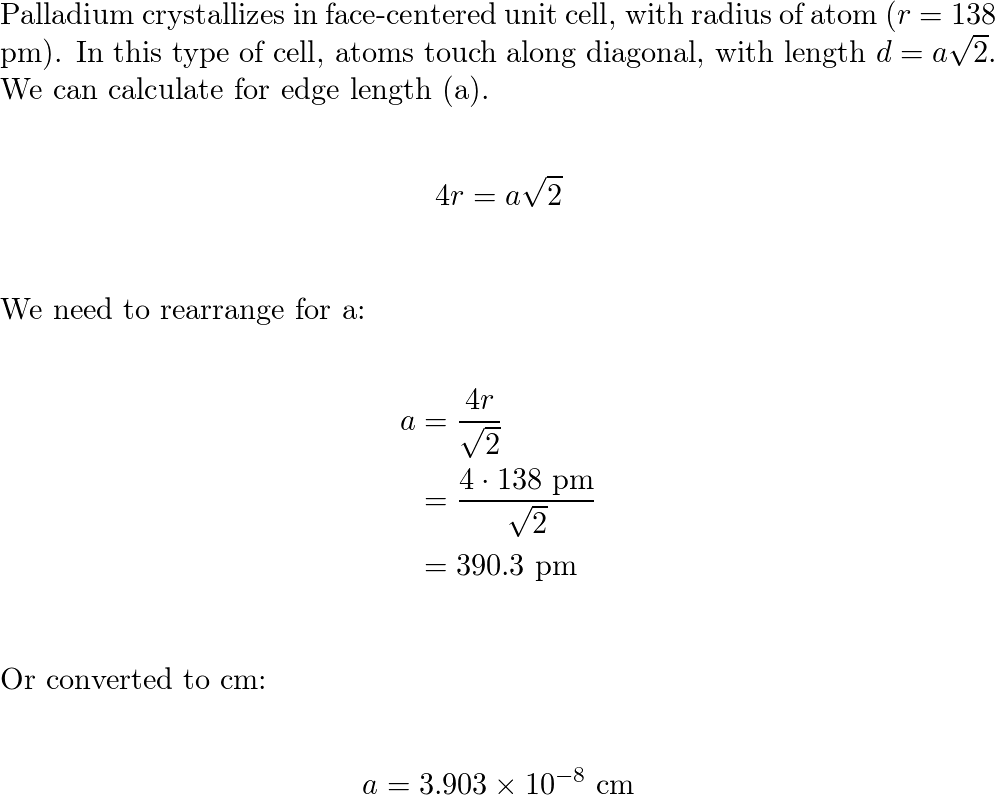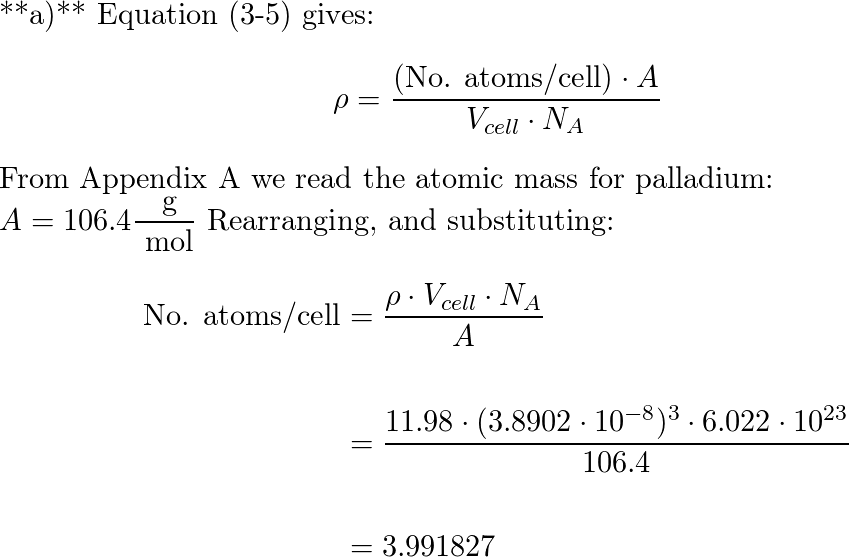SOLVED: Calculate the radius of a palladium atom in nm, given that Pd has an FCC crystal structure, a density of 12.0 g/cm3 and an atomic weight of 106.4 g/mol. A. 138

ASTM B984-12(2020)e1 - Standard Specification for Electrodeposited Coatings of Palladium-Cobalt Alloy for Engineering Use

Gold occurs as face centred cube and it has a density of 19.30 kg dm ^-3 .Calculate atomic radius of gold. (Molar mass of Au = 197 )

Gps Tracker 6 Meter Uns 2200 Grade 8.22 Gram Per Cubic Centimeter Density Rectangular Nickel Alloy Sheet at Best Price in Jamnagar | Vijay Laxmi Electro Platers